Rare Disease Is Anything But Average
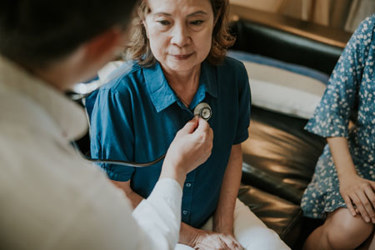
A countless number of notable figures from JFK to Marie Curie suffered from a rare disease defined in the U.S. as conditions affecting fewer than 200,000 people. With 7,000 to 10,000 known rare diseases and as many as 95% lacking approved treatments, the need for targeted research is urgent. However, recruiting patients for trials is a complex process. Diagnosis delays, limited awareness among healthcare providers, and geographical barriers all complicate patient identification and enrollment.
Cross-border clinical trials add additional layers of difficulty, including regulatory compliance, data privacy, and ethical considerations. Patients often need to travel long distances to access treatment, requiring customized support such as travel coordination, patient navigation, and financial reimbursement. These services, however, must be handled sensitively to avoid ethical pitfalls, such as undue influence or perceived coercion.
As the landscape of rare disease research evolves, so does the understanding that clinical trials must center on the lived experiences of participants. Humanizing research, providing adequate support, and engaging patients as true partners are crucial to advancing meaningful and impactful therapies. Explore how to advance rare disease research with a patient-first approach.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Clinical Leader? Subscribe today.
