Rethinking Recruitment Plans During The Pandemic
By Beth Harper
The unpredictable and unprecedented situation the coronavirus is creating is challenging all of us in so many ways … personally, professionally, economically, emotionally, and more. Each day that goes by, we learn that another biopharmaceutical company has made the incredibly difficult decision to delay the start of a new trial or halt enrollment for their existing trials. While less public, various online discussion groups and listserves portray the painful decisions that investigative sites are facing as their pipelines of trials and patient visits come to a screeching halt.
personally, professionally, economically, emotionally, and more. Each day that goes by, we learn that another biopharmaceutical company has made the incredibly difficult decision to delay the start of a new trial or halt enrollment for their existing trials. While less public, various online discussion groups and listserves portray the painful decisions that investigative sites are facing as their pipelines of trials and patient visits come to a screeching halt.
It’s not surprising that organizations are taking heroic efforts to divert their efforts and resources to address the pandemic, from repurposing laboratories to develop diagnostic tests, researching potential therapeutics for COVID-19, diverting clinical research staff to the front lines of patient care to other equally important activities. To say that this is wreaking havoc on clinical trial operations and study subject interactions is a gross understatement. Sponsors and CROs are quickly mobilizing to implement remote monitoring activities. Sites are scrambling to ensure the safe care and treatment of subjects, finding creative ways to distribute investigational products, and developing systems and processes to keep their staff and facilities virus-free. And, finding ways to operationalize decentralized clinical trials has taken on even greater importance.
The only thing that is certain is that the duration and impact this pandemic will have on the world of clinical research will be uncertain for the foreseeable future. I am optimistic that once the care and management of the current clinical trial participants have stabilized, we can find some silver linings in the pause. In that spirit, I am hopeful that as the dust settles, sponsors and CROs can use this opportunity to rethink their recruitment plans, support site sustainability, and intelligently prepare for the ultimate ramp up of trial enrollment.
Reflect On The Participant Supply Chain
If nothing else, the COVID-19 crisis has raised the visibility and collective understanding of supply chains. From personal protective equipment (PPE) to ventilators to healthcare providers, we have a greater appreciation for how the supply chain works and the serious ramifications that occur when it is broken. In my experience, the subject recruitment and participation process has always been a supply chain challenge. As such, the “leaky pipe” framework has been my tried and true method for depicting and understanding the recruitment process for 25 years. At its simplest form, supply chain management is about getting raw materials to a parts provider, through the production process and delivered to the customer. In our case, the raw materials are the subjects who need to be identified, get to the research sites, and be processed through the clinical trial in order to deliver the valuable data to the customer (sponsor). The leaky pipe visualization helps everyone understand whether the recruitment problem is about getting enough of the raw materials (subjects) into the system or “filling the funnel,” managing the leaks that happen along the way, or both.
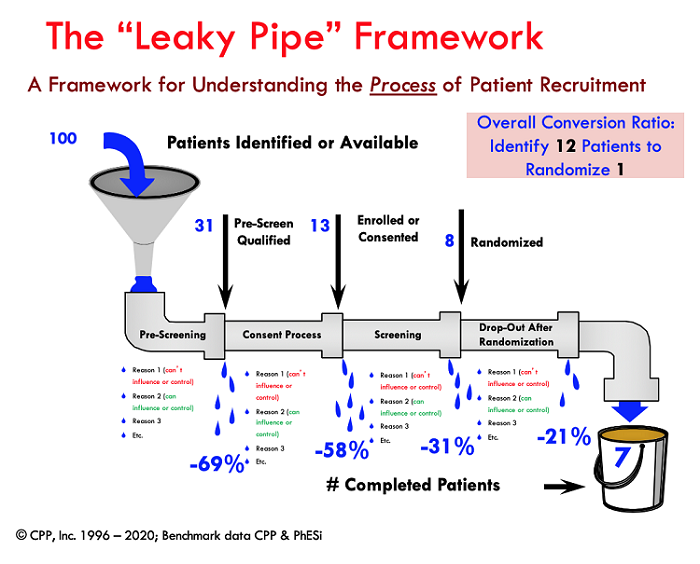
In most cases it is both, and benchmark data that I have been collecting along with support from my colleagues at PhESi (www.phesi.com) indicates that in general it takes about 12 available subjects to randomize one for a generic clinical trial. For rare diseases, the conversion ratio is about 28 to 1. Of course, this varies by the particular indication, with some trials having a much lower conversion ratio and other challenging trials having vastly bigger ratios. As a guidepost, however, these general parameters can be helpful for planning purposes. In other words, if a site is contracted to randomize eight subjects, they will need access to, or a supply of, about 100 subjects who have the core condition or diagnosis to fill the funnel. The conversion ratio can be optimized if the “leaks” can be minimized. Applying the ratio to the full study enrollment requirements provides insights as to how many overall subjects will need to be identified and can inform decisions about how many sites are realistically needed to recruit the required number of subjects.
Plugging The Leaks In The Pipe
By understanding both the loss ratios across the prescreening, consenting, and screening process as well as the reasons for the losses, sponsors can find opportunities to minimize the losses by adjusting the eligibility criteria, reducing study burdens, and enhancing the subject education process to decrease the likelihood that patients will fail or decline the opportunity to participate in the trial for preventable reasons. Naturally, some reasons for the losses can’t be influenced or controlled, but having an appreciation for why subjects are failing eligibility criteria, declining the opportunity to participate, or dropping out enables everyone to identify creative and ethically appropriate solutions to manage the leaks, which, in turn, requires fewer numbers of the supply of subjects at the top of the funnel.
With this in mind, the halt of enrollment provides an opportunity to reflect on the leaky pipe or recruitment funnel numbers. What are your current numbers telling you about the recruitment challenges and opportunities?
- Do you have access to enough subjects at the top of the funnel?
- Are your study awareness tactics working and, if not, are there ways you can reach more subjects by expanding your pool of sites or fine-tuning your recruitment methods and messages?
- If you are deploying specific campaigns, this is a good time to take a fresh look at the patient disposition metrics to determine what is and isn’t working.1
- Is this a time to revisit your inclusion/exclusion criteria to make some adjustments and amend the protocol?
- What interventions do you have in place to address the subject experience aspects?
- Can your subject education and support services and programs be enhanced by offering home visits, transportation support, or other ways to ease the burden of participating?
- What are high enrolling sites doing that could potentially be transferred to other sites?
If you don’t have the detailed data such as the top of the funnel numbers or the reasons for the leaks, can you work with your sites to capture this information with more granularity? Allocating some of the recruitment or study budget to have sites go back and evaluate this data creates an opportunity for them to keep some of their staff actively working, which, in turn, helps to alleviate their financial burdens and remain viable for the eventual ramp up in enrollment.
This is also a great time to get feedback from the sites on what is and isn’t working. Holding some virtual focus groups with the sites’ staff members can help keep them engaged and provide an opportunity to connect and share their experiences and recommendations, not only for how the recruitment and retention has worked thus far in the trial but to share feedback on new ideas, amendments, or new services you are considering. Systematically looking across all aspects of the recruitment funnel allows you to roll everything that might require ethics approval (e.g., amendment, new support services, materials) into one potential submission.
If you are just in start-up mode and sites haven’t actively started enrolling, can you use this opportunity to have them do more robust prescreening through EMR searches and chart reviews to start validating some of the leaky pipe numbers? Creating a simple financial agreement (if they are not already under contract) to reimburse sites for this work effort to identify and prescreen subjects (through their appropriate patient privacy and IRB/IEC policies, of course) to capture information about the loss ratios and reasons across the prescreening criteria can not only help sponsors develop more realistic enrollment projections, but it also creates another opportunity to support the sites financially.
If you are even earlier in the planning stages of a study that hasn’t started recruiting, could this pause in activity be an opportunity for you to research and develop a more robust patient pathway and overarching patient recruitment plan?2, 3 These are two incredibly important and essential factors for recruitment success that are often given short shrift in the typical flurry of study launch activities.
Of course, no one knows how clinical trials will change as a result of the pandemic. We could see drastic changes to protocol designs, healthcare delivery, subject willingness to participate, the clinical research workforce, and more, all of which could have far-reaching implications for how we recruit and retain subjects in clinical trials. Despite this uncertainty, working with what we do know — our current recruitment plans and leaky pipe metrics — may provide some sense of normalcy and control that many of us are craving.
However you are able to weather the coronavirus craziness, here’s to your good health and to all of us reemerging with more effective and efficient clinical trial processes than ever.
References:
- Harper, B. Do Patient Recruitment Advertising & Awareness Campaigns Really Work? https://www.clinicalleader.com/doc/do-patient-recruitment-advertising-awareness-campaigns-really-work-0001
- Harper, B. Precise Patient Recruitment Planning – It's All About The Patient Pathway.https://www.clinicalleader.com/doc/precise-patient-recruitment-planning-it-s-all-about-the-patient-pathway-0001
- Clinical Performance Partners, Infographics: http://clinicalperformancepartners.com/infographics/
About The Author:
 Beth Harper is the president of Clinical Performance Partners, Inc., a clinical research consulting firm specializing in enrollment and site performance management. She also is the workforce innovation officer for the Association of Clinical Research Professionals (ACRP). Harper has passionately pursued solutions for optimizing clinical trials and educating clinical research professionals for over three decades. She is an adjunct assistant professor at George Washington University who has published and presented extensively in the areas of protocol optimization, study feasibility, site selection, patient recruitment, and sponsor-site relationship management. Harper serves on the CISCRP Advisory Board and the Clinical Leader Editorial Advisory Board. She can be reached at 817-946-4728, bharper@clinicalperformancepartners.com, or bharper@acrpnet.org.
Beth Harper is the president of Clinical Performance Partners, Inc., a clinical research consulting firm specializing in enrollment and site performance management. She also is the workforce innovation officer for the Association of Clinical Research Professionals (ACRP). Harper has passionately pursued solutions for optimizing clinical trials and educating clinical research professionals for over three decades. She is an adjunct assistant professor at George Washington University who has published and presented extensively in the areas of protocol optimization, study feasibility, site selection, patient recruitment, and sponsor-site relationship management. Harper serves on the CISCRP Advisory Board and the Clinical Leader Editorial Advisory Board. She can be reached at 817-946-4728, bharper@clinicalperformancepartners.com, or bharper@acrpnet.org.
