Trends In Rare Disease Trials: Evaluating Trial Types' Pros, Cons, And Adoption Rates
By Sapna Rani, lead analyst, Beroe Inc.

In the evolving field of rare disease research, the strategic choice of clinical trial design is fundamental to achieving robust scientific validity, regulatory approval, and operational feasibility. This article, the second in our series (read the first here), provides a comprehensive evaluation of leading trial types from traditional RCTs and single-arm studies to adaptive, basket, micro trials, and registry-based models tailored for rare disease development. We compare adoption rates, design-specific trade-offs, and practical selection criteria, preparing for our next article, which will provide a strategy-focused framework and actionable recommendations for rare disease sponsors.
Criteria For Evaluating Solutions
To effectively judge new approaches to rare disease trials, stakeholders should apply these criteria:1,2
- Patient Centricity: Solutions must demonstrate tangible impact on identifying, engaging, and retaining patients from highly dispersed and ultra-small populations. Success is measured by the ability to harness registries and RWD for dynamic feasibility assessment, support multilingual content, enable decentralized participation (telemedicine, home care, etc.), and provide transparent communications with both patients and their advocacy groups.
- Regulatory Acceptability: Strong candidates proactively support regulatory intelligence offering pre-submission advisory, compliance alerts, harmonization across regions (FDA, EMA, PMDA, etc.), and seamless reporting for orphan drug or special pathway applications. Evaluate if the provider/platform can demonstrate successful rapid interactions with multiple health authorities and their adaptation to RWE acceptance, post-approval surveillance, and risk management.
- Operational Feasibility: Given the inherent limitations of rare disease research, platforms and CROs must support adaptive, Bayesian, and nontraditional study designs (e.g., N-of-1, basket, platform, crossover, or delayed start). Evaluation includes whether solutions facilitate interim analyses, protocol amendments, external control use, and statistical tools that optimize small-sample efficacy inference.
- Scientific Rigor: Evaluate the solution’s approach to defining and validating patient-centric outcome measures (e.g., clinical outcome assessments [COAs], patient-reported outcomes [PROs], and biomarker endpoints). The ability to tailor endpoints to heterogeneous presentations, use disease-specific scales, and document FDA/EMA engagement (e.g., via RD-COAC (Rare Disease Clinical Outcome Assessment Consortium) workstreams is essential.
- Scalability: Given the multi-center, multi-country nature of rare disease trials, solutions must reliably scale from pilot micro-trials to global registries. Assessment criteria include cloud-based architecture, modular functionality, open APIs, integration capacity with biobanks, companion diagnostics, and patient ID systems. Uptime, disaster recovery, and support quality are crucial for sustained trial integrity under variable conditions.
- Sustainability: Ultimately, solutions should be evaluated for their contribution to accelerating approvals, reducing timelines, lowering per-patient costs, and producing meaningful, generalizable patient outcomes. Evidence of long-term sustainability, robust business model, ongoing ecosystem partnership, and continuous innovation offers reassurance that the provider/platform will grow with the needs of the rare disease research community.
Solutions & Proposed Approach
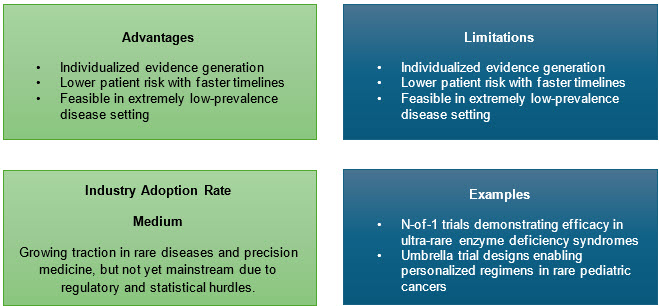
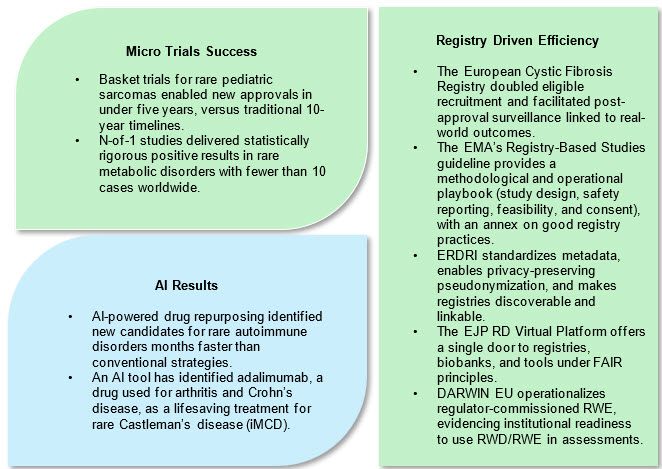
1. Micro-Trials and Innovative Adaptive Protocols
Micro-trials including n-of-1, Bayesian adaptive, and umbrella studies are especially suited for rare diseases. By focusing on strong mechanistic hypotheses and robust phenotyping, they enable statistically sound inference from ultra-small samples. Adaptive elements allow mid-trial reallocation of resources, sample size adjustments, or accelerated expansion when positive signs appear.3
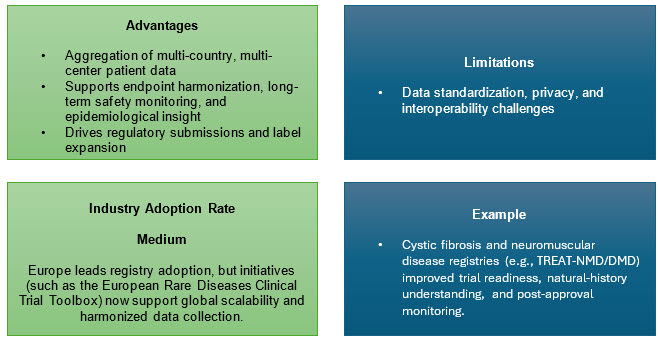
2. Global Patient Registries
International registries provide the backbone for rare disease research, compiling natural history, genotype/phenotype, and treatment/outcome data in real time, which is vital for drug development, post-market surveillance, and understanding disease heterogeneity.
In the U.S. and globally, patient-owned data platforms like RARE-X (Global Genes) enable standardized, shareable data sets governed by patient consent, an emerging route to scalable, cross-disease, cross-border research.
RWE at regulator scale: The EMA’s DARWIN EU network demonstrates how federated EHR/registry data can deliver RWE throughout the product life cycle — a template for post-authorization safety/effectiveness and for contextualizing trial outcomes.4
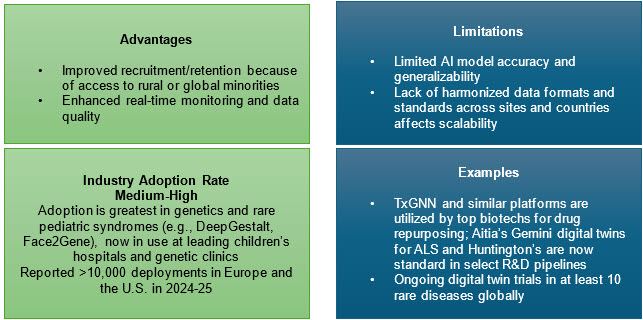
3. AI and Digital Technologies
AI tools accelerate diagnostic screening, patient identification, phenotype classification, and molecular target discovery. AI algorithms such as DeepGestalt and network-based drug repurposing platforms have expedited trial recruitment, improved clinical endpoint selection, and even identified new therapies for conditions like Castleman's disease.
Digital innovations also streamline e-consent, wearable data collection, and virtual siteless trials, greatly reducing patient burden and widening access, especially for remote populations. Regulators in the U.S. and EU now provide clear expectations for oversight, data integrity, safety, and the role of local HCPs. For geographically scattered rare-disease communities, hybrid designs may be the difference between feasible and infeasible recruitment.5
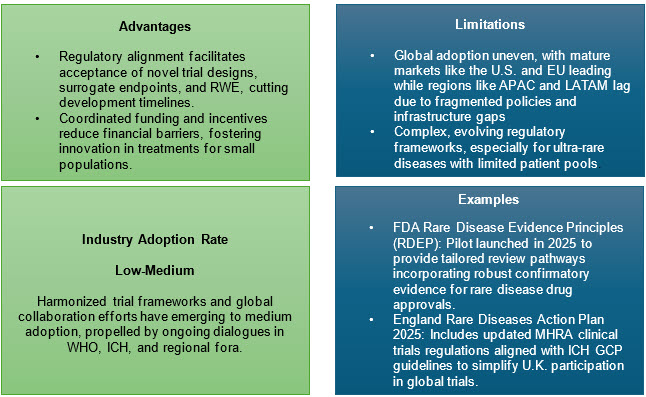
4. Policy, Funding, And Regulatory Harmonization
The 2025 World Health Assembly’s global action plan emphasizes registry investment, digital infrastructure, and international consortia to advance research, harmonize standards, and reduce treatment gaps within a 10-year framework. Targeted funding initiatives (e.g., orphan drug grants and rare disease priority review vouchers) incentivize development and lower economic barriers.
International regulatory harmonization aligns data standards, approval routes, and ethical oversight, promoting efficiency and scale.6
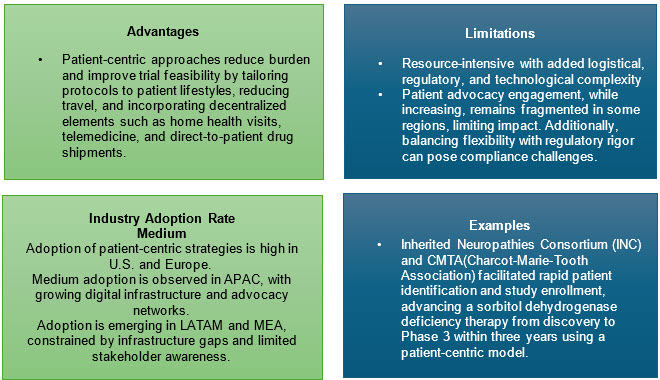
5. Patient-Centric And Equitable Strategies
Active engagement of patients and caregivers in study protocol design, digital feedback, and operational logistics maximizes retention and relevance. Siteless trials, home nurse visits, and flexible protocol designs minimize participant burden, particularly for pediatric and under-resourced families.
Inclusiveness must extend to economic support, multi-lingual resources, travel subsidies, and caregiver involvement in data collection.
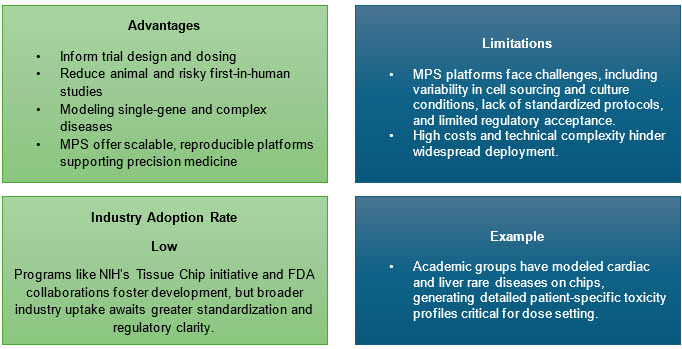
6. Integration Of Microphysiological Systems(MPS), Or Clinical Trials-on-Chip
Advances in organ-on-chip and tissue chip models provide preclinical platforms for rare diseases, allowing efficacy and safety screening without relying solely on small patient pools.
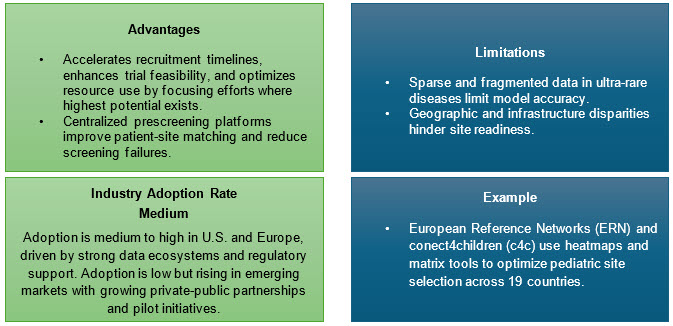
7. Data-Driven Site And Patient Identification
Leveraging RWD, genomic profiling, and AI algorithms for site selection is supplanting legacy CRO methods. Collaborations with patient advocacy organizations and global data platforms (e.g., RARE-X, GRDR) facilitate direct recruitment and disease awareness.7,8
Approaches:
- Utilize large EHR and claims data to locate eligible patients.
- Build and link registry databases, enabling protocol eligibility matching
Supporting Analysis & Evidence
Rare Disease Growth Trends
The global rare disease market is booming, with the sector expected to grow at a CAGR of 11.93% from 2025 to 2030, with the market expected to be $242.5 billion in 2025. Peak activity is seen in oncology (72%), followed by immunology (10%) and cardiovascular diseases (5%).9
The number of registered rare disease clinical trials has shown consistent growth from 2020 to 2025, driven by increased investment, regulatory incentives, and advancements in precision medicine and digital health technologies.
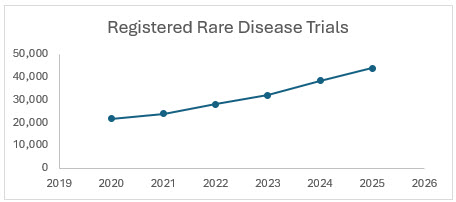
Sources: Clinicaltrial.gov, citeline
In 2024, 52% of new FDA approvals were for rare diseases, suggesting rising registration and R&D prioritization for this segment, and 54.2% of rare disease trials were Phase 3, showing increased focus on late-stage, pivotal studies. From 2020 to 2025, Asia Pacific rare disease trials grew at a CAGR of 8.4%. The rare disease clinical trial registration market CAGR 2020–2025 averages 6.8%, accelerating late in the period due to new therapies and advanced designs.9
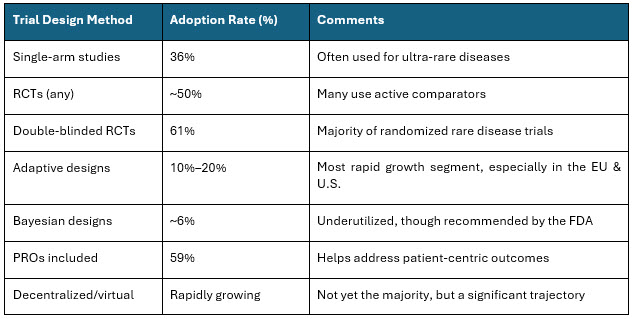
Table 3: Trial Design Adoption Rate
Sources: https://pmc.ncbi.nlm.nih.gov/articles/PMC10476732/, https://pmc.ncbi.nlm.nih.gov/articles/PMC12372023/10
The rare disease clinical trial sector is expanding quickly, with method adoption moving toward decentralized, adaptive, RWE, and patient-driven models, while conventional RCTs and single-arm studies are still most common. Bayesian and advanced analytical approaches are recommended but not yet widely used.
Conclusion
The landscape of rare disease clinical trials continues to transform through innovation in trial design and regulatory evolution. No single trial design fits all rare diseases; instead, an array of approaches, including micro trials, adaptive frameworks, basket and platform trials, and registry-based studies, offer flexible solutions to the operational and scientific challenges unique to this field. Increasing regulatory openness to patient-centric, real-world, and biomarker-driven endpoints marks a paradigm shift, empowering sponsors to develop effective therapies efficiently while addressing ethical imperatives. Going forward, a strategic, evidence-driven approach to trial design selection, balancing rigor, feasibility, and patient needs, will be essential.
In the third and final article of this series, we will present a practical framework for trial design selection in rare disease research, sharing solution pathways, partnership strategies, and innovation-driven recommendations for the decade ahead.
References:
- M. M. O. M. J. P. B. B. C. M. a. R. K. M. Vivek Subbiah, "Designing Clinical Trials for Patients With Rare Cancers: Connecting the Zebras," ASCO Publications, 2025.
- "Exploring the Key Types of Clinical Trial Designs in Modern Research," 2025. [Online]. Available: https://comac-medical.com/exploring-the-key-types-of-clinical-trial-designs-in-modern-research/.
- N. B. 2. J. C. 2. L. Z. 3. C. S. 4. A. F. 2. Dawn Edwards 1, "Using Bayesian Dynamic Borrowing to Maximize the Use of Existing Data: A Case-Study," pubmed, 2023.
- "Rare Disease Clinical Trials: Challenges, Strategies, and Solutions for Success," 2023. [Online]. Available: https://www.allucent.com/resources/blog/rare-disease-clinical-trials-challenges-strategies. [Accessed 2025].
- "Rare Diseases Clinical Trials Toolbox," 2025. [Online]. Available: https://ecrin.org/rare-diseases-clinical-trials-toolbox.
- "World Health Assembly adopts first-ever resolution on rare diseases, signalling a new era of global collaboration," May 2025. [Online]. Available: https://erdera.org/news/world-health-assembly-adopts-first-ever-resolution-on-rare-diseases-signalling-a-new-era-of-global-collaboration/. [Accessed 2025].
- "AI identifies life-saving treatment for rare Castleman’s disease," Feb 2025. [Online]. Available: https://www.drugtargetreview.com/news/156022/ai-identifies-life-saving-treatment-for-rare-castlemans-disease/. [Accessed 2025].
- S. M. 2. K. A. 3. M. C. 4. R. C. G. 5. E. C. K. 6. M. S. 7. Kristen Wheeden 1, "Enhancing and leveraging principal investigator and patient advocacy group collaboration in rare disease clinical research-meeting report from the rare Diseases Clinical Research Network," Pubmed, 2025.
- "Citeline-Rare Disease R&D: Continued Growth Amid Challenges," Feb 2025. [Online]. Available: https://www.citeline.com/en/resources/rare-disease-r-and-d. [Accessed 2025].
- M. B. 2. M. G. 2. A. A. 2. A. M. 3. P. L. C. 1. F. L. 2. C. L. 2. Claudio Jommi 1, "Pivotal Studies for Drugs About to Be Launched for Rare Diseases: Will They Better Support Health Technology Assessment and Market Access than in the Past?," Pubmed, 2025.
About The Author:
Sapna Rani is a lead analyst, pharma R&D – clinical and preclinical research with over nine years of experience in market research and consulting. Her insights have enabled top pharma companies in their strategic decisions on supplier outsourcing, category management, and planning. In the past year, she was engaged in more than 12 market sourcing studies, five supplier data visualizations, and multiple quick reactive analysis across clientele for global and regional requirements.
