Trends In Rare Disease Trials: Trial Designs, Challenges, And Regulatory Progress
By Sapna Rani, lead analyst, Beroe Inc.

A rare disease, as defined by the World Health Organization, affects fewer than one person in every 1,000, and the cumulative burden is enormous, with more than 7,000 distinct conditions identified to date. Despite this, 95% of rare diseases lack FDA- or EMA-approved treatments. Patients typically face years-long journeys to diagnosis, fragmented expertise, and limited access to research trials. Pediatric prevalence is high; around half of rare disease patients are children, making ethical and logistical trial design a particularly complex challenge.1
The rare disease clinical trial ecosystem is rapidly evolving in response to scientific, operational, and regulatory complexities. A shift from classical randomized controlled trials (RCTs) to innovative, flexible model including micro trials, adaptive designs, registry-based studies, and n-of-1 approaches reflects both the constraints and the opportunities inherent in studying small, heterogeneous patient populations. Regulatory authorities have responded with progressive frameworks, incentives, and methodological flexibility. This article reviews the evolution of trial design types in rare disease research, analyzes ongoing challenges, and examines how regulatory support is enabling innovation, preparing for a follow-up discussion on how sponsors can match trial design to research needs and emerging solutions.
Rare diseases, though individually infrequent, collectively affect more than 300 million people worldwide. Clinical research for these diseases is confounded by ultra-small patient populations, diagnostic uncertainty, and a lack of natural history data or robust clinical endpoints. The landscape of rare disease trials has expanded from traditional methodologies to incorporate patient-centric and data-driven innovations. Registries, micro trials, and adaptive and decentralized models are increasingly key in generating actionable clinical evidence. Regulatory agencies, recognizing the urgent unmet needs and complexities of this space, have implemented novel supports and expedited pathways, changing how trials are designed and reviewed.1
Evolution Of Trial Designs
Historically, the randomized controlled trial (RCT) has served as the gold standard for drug development. However, rare disease trials often struggle with low patient numbers, variable phenotypes, slow recruitment, and poorly defined endpoints.
In response, the past five years have seen a pivot toward:
Micro-trials/N-of-1 Studies
- These are experimental, highly personalized, and not yet standardized.
- Emerging digital biomarkers and wearables could push them forward, but today they remain at the earliest maturity level.
Basket & Umbrella Trials
- These are widespread in oncology (close to matured) but in rare diseases are still growing due to lack of validated biomarkers.
- This is a transitional growth model with strong potential but uneven maturity across therapy areas.
Synthetic Control Arms
- Already embedded in regulatory pathways (especially in the U.S.) and increasingly adopted by pharma/biotech.
- Mature enough to be mainstream, though regional regulatory differences remain a limiting factor for global standardization.
Global Patient Registries
- Broadly established, especially in neurology and rare diseases.
- Critical for recruitment and post-marketing, but fragmented and not yet harmonized globally, hence straddling growth and matured.
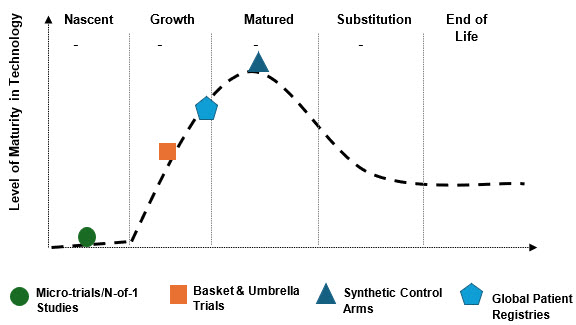
Figure 1 – Rare Disease Trial Maturity Curve
Source: Beroe Analysis
Together, these innovations, supported by advances in AI for patient phenotyping and drug discovery, as well as evolving policy frameworks, such as the recent World Health Assembly rare disease resolution, are transforming the landscape of rare disease clinical research.2
Digital & Policy Shifts
Regulators on both sides of the Atlantic have responded with guidance that explicitly anticipates small populations, alternative designs, use of real-world data (RWD), and registry-based studies. The EU’s “Clinical Trials in Small Populations” guideline formalized design flexibilities as far back as 2006 and remains foundational. The International Council for Harmonisation (ICH) has strengthened the statistical backbone through E9(R1) Estimands (finalized in 2019–2021), improving clarity around treatment effects when intercurrent events and small samples complicate inference.
Global digital infrastructure, telemedicine, e-consent, and wearable devices lower barriers to entry, particularly for remote and underrepresented patients. Policy changes, including the recent World Health Assembly resolution, signal increased international commitment to harmonized research, funding, and equitable access.3
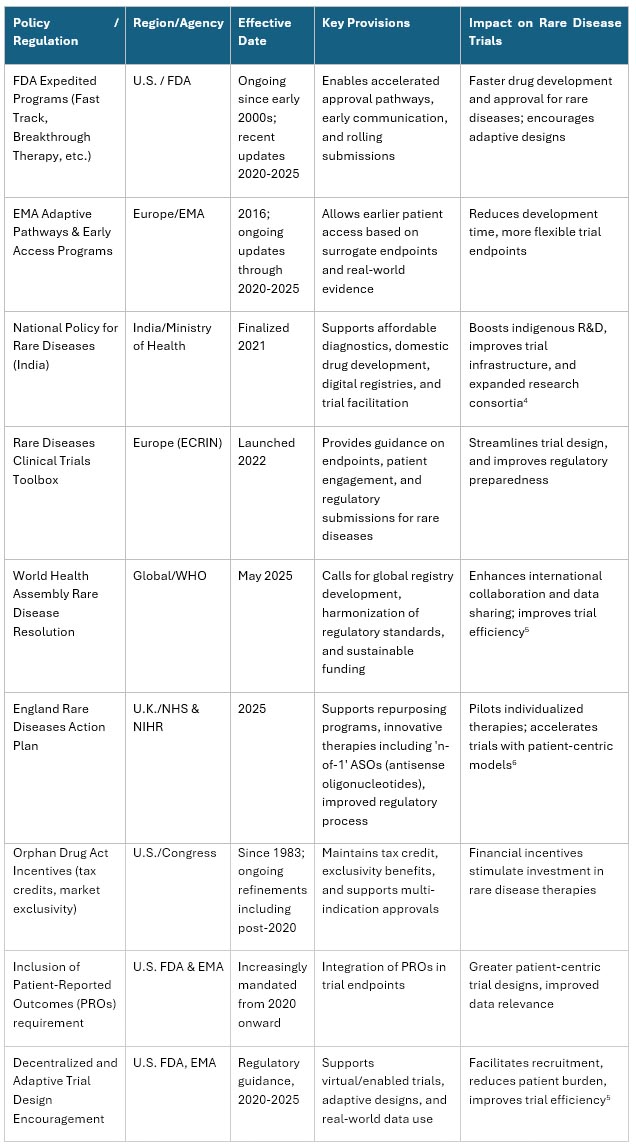
Table 1 – Policies Impacting Rare Disease Trials
Sources: https://www.gov.uk, https://erdera.org/, U.S. FDA, https://icer.org/, https://rarediseases.mohfw.gov.in/
These digital and policy shifts collectively aim to transform rare disease trials into more adaptable, patient-centric, and efficient processes, accelerating treatment discovery and accessibility worldwide by 2025 and beyond.
Problems Persist
Despite progress, rare disease trialists continue to face enduring structural obstacles. Table 2 below highlights the key challenges in rare disease trials.
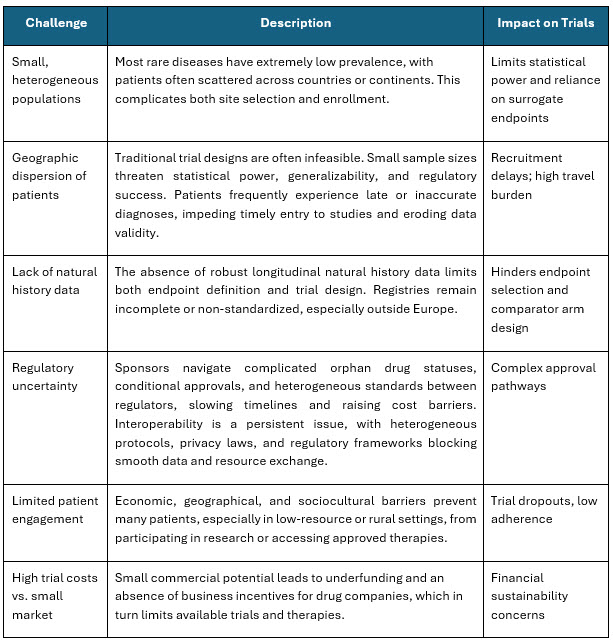
Table 2 – Challenges in Rare Disease Trials
Sources: https://pubmed.ncbi.nlm.nih.gov/40079686/, https://pmc.ncbi.nlm.nih.gov/articles/PMC3964003/
These issues drive high trial failure rates and long timelines, limiting therapeutic access for vulnerable populations.
Conclusion
The future of rare disease trials is defined by methodological innovation, regulatory agility, and increasing reliance on patient-centric real-world evidence. The shift from large, parallel-arm RCTs to micro trials, adaptive models, and registry-based studies marks a fundamental change in how rare disease therapies are developed and assessed. These innovations are not just technical; they are necessary responses to clinical, operational, and ethical realities that will only intensify as genomics and personalized medicine advance.2
In the next part of this series, we will discuss both the practical criteria and the cutting-edge solutions for matching the right trial design to the needs of each rare disease program.
References:
- "Trends in orphan medicinal products approvals in the European Union between 2010–2022," 2024. [Online]. Available: https://pmc.ncbi.nlm.nih.gov/articles/PMC10900541/. [Accessed 2025].
- M. V. 1. S. S. 1. G. F. 1. O. I. 2. Fran Brown 1, "Impact of changes in regulatory framework on approval of medicines for rare diseases and applicability to market access policies," Frontiers in Medicine, 2025.
- "Rare Diseases at FDA," 2025. [Online]. Available: https://www.fda.gov/patients/rare-diseases-fda.
- "Digital Portal for Crowdfunding & Voluntary donations for Patients of Rare Diseases," [Online]. Available: https://rarediseases.mohfw.gov.in/.
- "Advancing rare disease research: Exploring opportunities for Bayesian methods with FDA’s upcoming guidance," 2025. [Online]. Available: https://www.parexel.com/insights/blog/advancing-rare-disease-research-exploring-opportunities-for-bayesian-methods-with-fdas-upcoming-guidance.
- "England Rare Diseases Action Plan 2025: main report," 2025. [Online]. Available: https://www.gov.uk/government/publications/england-rare-diseases-action-plan-2025/england-rare-diseases-action-plan-2025-main-report.
About The Author:
Sapna Rani is a lead analyst, pharma R&D – clinical and preclinical research, with over nine years of experience in market research and consulting. Her insights have enabled top pharma companies in their strategic decisions on supplier outsourcing, category management, and planning. In the past year, she was engaged in more than 12 market sourcing studies, five supplier data visualizations, and multiple quick reactive analysis across clientele for global and regional requirements.
