Unlocking The Patient Voice: How Digital Data Capture Is Transforming Healthcare Decision Making
By Tim Davis, Vice President, Digital Patient Solutions, ERT
Introduction
Assessing the value and effectiveness of new drugs is essential in the pharmaceutical industry, but how these important decisions are made has undergone remarkable change in recent years, alongside huge advances in technology. Only thirty years ago, new drugs were assessed mainly on their commercial value, with the focus shifting in the following years toward cost- and clinical-effectiveness. Since then, Real-World Evidence (RWE) and listening to the patient voice have become much more important.
Today, RWE and humanistic patient generated outcomes are key to guiding all stakeholder decisions, including regulators, physicians, payers, and patients themselves. For example, in a recent ISPOR survey, almost 90% of payers reported that they use RWE in decision making1. Much of this is due to the recognition that the gold standard of new drug assessments, traditional randomized controlled trials (RCTs), operate in idealized environments. They also measure efficacy in limited patient groups and thus cannot predict the effectiveness of new treatments in real-world settings.
Companies are increasingly implementing supplementary real-world programs, in which large groups of patients report their own health outcomes in real life settings over long durations. The resulting Real-World Data (RWD) feeds into RWE to inform decision makers about how effective new drugs are for patients as they go about their daily lives.
However, capturing RWD directly from patients can be challenging, and these challenges could be hindering the widespread adoption of RWD programs that would otherwise be valuable. Fortunately, new technological solutions are overcoming these challenges, enabling humanistic patient reported outcomes (PROs) that enhance RWE and support better informed healthcare decisions.
Using technology to overcome the challenges of RWD capture
The challenges of capturing PROs in RWD programs largely arise from the use of paper-based data capture methods. These involve the regular completion of paper forms by patients, which they subsequently send to program organizers to be transcribed into a central database. This manual effort presents various challenges to both patients and manufacturers.
Alternative electronic data capture platforms, and the use of patients’ own devices through a ‘Bring Your Own Device’ (BYOD) approach, offer several solutions to the challenges of paper-based data capture (Table 1).
Table 1: Challenges of paper-based RWD capture methods and the solutions offered by electronic data capture.
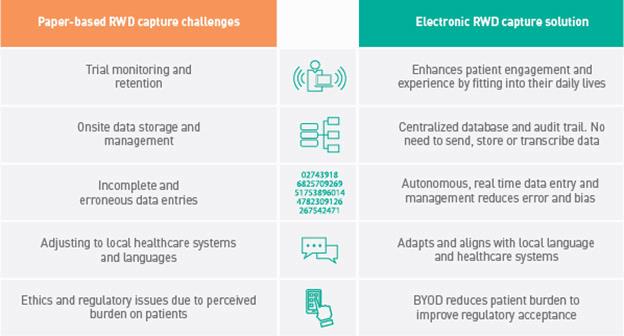
Despite their significant advantages, electronic data capture platforms have not been adopted by RWD program organizers as widely as would be expected. This is most likely because manufacturers perceive the initial cost of provisioning electronic devices to large patient groups as too high to justify. However, a BYOD approach enables the cost-effective digital capture of high-quality RWD to potentially support significant returns on initial investment.
Applying technology to RWD programs
The adoption of electronic platforms shows great promise in overcoming the challenges of RWD capture, including in observational studies, pragmatic trials and commercial programs. Each setting involves its own challenges, which are successfully addressed by technological solutions.
Observational studies
In observational studies, a group of patients is monitored over an extended period to assess the effects of a new drug on health outcomes. By definition, these studies are non-interventional, with investigators only recording what happens to patients; no diagnostic or monitoring procedures are applied. As such, capturing humanistic PROs commonly forms an important part of the study design.
A major challenge presented by observational studies is the potential for burden to be placed on patients due to them having to regularly complete paper forms, such as questionnaires. When designing an observational study, the burden on patients must be carefully balanced; too little may impair the integrity of the data, while too much may influence patient outcomes and impact ethical approval.
An electronic approach enables patients to use their own devices in observational studies, so manufacturers can cost-effectively integrate electronic platforms into patients’ daily lives. This represents a very low level of patient support to minimize intervention and assist with approval by ethics committees.
Electronic platforms are also easily adaptable to different countries’ healthcare systems as well as to local languages, enabling patients to input their data wherever they are in the world. Additionally, electronic platforms enable the implementation of data protection software to ensure compliance with data protection and privacy conditions in clinically validated environments.
Pragmatic trials
Pragmatic trials involve assessing the effectiveness of treatment interventions in broad routine clinical practice conditions (unlike explanatory trials, which assess the intervention in a well-defined and controlled setting). Pragmatic trials have recently experienced a resurgence, generating data that can be generalized and applied in real-life settings to feed into RWE. The growing importance of these trials is currently fostering the development of new supportive initiatives and tools. This includes the PragMagic tool, which assists with pragmatic trial design and protocol evaluation and will facilitate the continued generation of valuable RWD.
Electronic data capture solutions are well-suited to supporting pragmatic trials, particularly through a BYOD approach, because they can ensure the capture of reliable PROs and completion of health-related quality of life assessments. The increase of electronics in patients’ day-to-day lives, such as smartphones, mobile devices and consumer apps, means they expect to engage with similarly intuitive and user-friendly services during trial participation.
Electronic platforms easily fulfil these expectations. They have a broad range of capabilities to facilitate engagement and interaction with participants in a regulatory-compliant manner. For example, a scripted chat box that meets ethics requirements can be incorporated into the platform to assist patients with queries and provide supporting information. The platforms can also be designed to gather patients’ answers to validated quality of life questionnaires at ad hoc time points, as well as provide educational support tools such as videos about their medical condition. Moreover, these platforms can be integrated into patients’ other devices, such as activity monitors, as part of the RWD capture solution.
While some or all of these capabilities can be deployed in electronic data capture platforms for pragmatic trials, program organizers can work with their technology partner to assess what each trial requires on a case-by-case basis, in order to balance internal validity versus real-world effectiveness. As the electronic platforms minimize data entry errors, study teams can be assured that the RWD generated will provide high quality RWE (with a full audit trail) to facilitate regulatory approval.
Commercial programs
Pharmaceutical companies are increasingly implementing commercial patient support programs to improve and manage their treatment in real-world settings. These programs are deployed once a new drug has been licensed and is available on the market, and when patients require further support while they take the treatment. For example, commercial programs can be set up to support patients’ adherence to treatments when they are dealing with severe side effects.
While the company’s primary goals are to enhance patient support and protect their brand, one of the by-products of commercial programs is the generation of humanistic patient reported RWD, which can contribute to the wider RWE base to inform healthcare decision making.
One example of a commercial program being successfully supported by an electronic data capture platform is TARGET My Hives, a digital health network for everyone living with and impacted by chronic urticaria. The 3,500-strong community allows patients to connect with each other electronically, as well as with physicians and manufacturers, to reduce feelings of isolation, seek medical advice, and contribute to surveys that generate further understanding of chronic urticaria. The information generated by this online community is providing a rich source of data that could further support RWD programs.
Conclusion
The practice of ensuring that new drugs are safe and effective has undergone dramatic change in recent years. Today, all healthcare stakeholders, including regulators, payers and patients, are placing increasing importance on the patient voice in informing their decisions about the effectiveness of new drugs in real life settings. This is encouraging the pharmaceutical industry to capture RWD, to feed into RWE and support healthcare decisions based on humanistic PROs.
Current RWD programs using paper-based RWD capture methods place a significant burden on both patients and manufacturers, but proven technological solutions can overcome these challenges. Electronic RWD capture platforms, together with the use of patients’ own devices, are enabling the cost-effective capture of high quality RWD in observational studies, pragmatic trials, and commercial programs.
Effectively unlocking the patient voice in these RWD programs by using electronic platforms is a straightforward process that involves defining and implementing the technological solution on a case-by-case basis. This will ensure regulatory/ethical compliance, as well as effective RWD capture in line with the program’s specific goals.
Once the technological solution is in place, companies will benefit from more efficient, cost-effective and reliable RWD capture and RWE generation compared to traditional paper-based RWD capture methods. Electronic capture solutions are presenting a future where the burden of RWD capture on patients and manufacturers is significantly reduced, meaning we can start to fully leverage the potential of the patient's voice in informing healthcare decision making.
References
- Use of Real-World Evidence in Payer Decision-Making: Fact or Fiction? ISPOR Issue Panel. May 23, 2016.
As originally seen in Journal of mHealth
