Why All The Talk About Real-World Evidence?

By Ed Miseta, Chief Editor, Clinical Leader

Real-world evidence (RWE) has become a topic everyone wants to discuss. Several executives I have spoken to recently believe it will continue to grow in importance. But why the sudden interest in RWE?
Brian Cuffel, VP and market access head of oncology at Bayer, and William Daley, VP of cardiovascular thrombosis for Sanofi U.S., see several factors driving the interest. First, regulators, namely the FDA and European Medical Agency, are paying more attention to RWE than ever before. Both men believe the impact of RWE will be seen in the pre-approval process where this data will help to cut drug development timelines.
“By using RWE, regulators can really accelerate novel agents through the approval process and into the hands of patients,” says Cuffel. “RWE supports their decision making in a way that was simply not possible in the past. It’s an important new development, in oncology in particular, and, as such, interest in it is likely to grow.”
Daley adds that RWE is a perfect complement to the data already generated during clinical trials. Although, he cautions that this trend is still in its infancy and notes that even with all of the current excitement and enthusiasm over RWE, it will take more time for it to make an impact on regulatory approvals.
Are Your Products Truly Effective?

“Clinical trials are not always applicable to the general population,” he says. “Inclusion and exclusion criteria will keep many patients from being able to participate in a trial, due to the need for a clean population that will not introduce bias into the study. But once the product is made available to the general population, we can really begin to gauge its effectiveness. Understanding the effectiveness of new products at this stage is of paramount concern for both regulators and sponsors, which is driving the trend of collecting more RWE.”
Daley says being able to see the impact of a drug on people in their everyday lives — how they take the product, what it does and doesn’t do — all of that can be very different from what you see in a controlled population in a clinic.
Technology advancements in drug development also are sparking interest in RWE. Cuffel notes advanced healthcare analytics coming from IBM’s Watson and companies such as Google are helping to transform the industry. For example, IBM’s Watson will enable companies to integrate data from all sources in the clinical trial ecosystem, including EMRs, healthcare professionals, patients, data managers, and sites. Additionally, insurance companies, particularly in the U.S., are looking for medicines that can demonstrate improved patient outcomes and reduce hospitalization rates and length of stays. There is also an industrywide effort to bring more personalized medicines to market. All of those efforts are generating valuable data and creating a strong argument for adoption of RWE in more trials.
“Payers want to understand the effectiveness of a new drug on the general population, so they gain better insights into what they are paying for,” says Daley. “Payers need to understand the benefit to patients, as well as which patients are most likely to benefit from the treatment. Even policymakers will look at the results of trials to make decisions.” In short, there is almost no stakeholder in the drug development process who would not benefit from the additional insights that can be gained from RWE.
How Do We Gather The Data?

“In oncology, evidence leading to regulatory approval can sometimes be an extension to a Phase 1 study,” says Cuffel. “RWE can be very helpful in providing access to historical controls on standard of care. By combining RWE with new data we have on our molecule, we can help accelerate that regulatory decision-making process. The process of RWE generation can then be extended into the post-approval phase, subsequent to a conditional approval of the molecule. This will help increase patient access to the medicine while companies continue to generate additional RWE on the safety and efficacy of the drug.”
Cuffel and Daley agree that gathering the evidence prior to regulatory approval is done via a combination of clinical trials and early-access programs. The goal of RWE is to generate comparative data on standard of care and to demonstrate how the new treatment being advanced is better than what is currently on the market. That makes Phase 1 and Phase 2 trials a good place to gather additional data that could assist with approval or even planning of the Phase 3 trial.
Diversity is one key factor outlining the importance of gathering RWE early. In a clinical trial, it is often difficult to predict what the effect of a drug will be on patients of diverse genders and ethnic backgrounds. The reason for this is the lack of representation in trials of many of those groups.
“Even in a clinical trial with 15,000 or 20,000 patients, you might be lucky to have a couple hundred individuals of Hispanic or African American descent,” says Daley. “It would be difficult to make any statement about the effect on those 200 patients in a 20,000 patient study. Having RWE is a much better way of determining a product’s effectiveness in various ethnic populations. With RWE, we have a much more robust data set from which conclusions can be drawn.
A Perfect Match With Patient-Centricity And Big Data
As companies begin to gather RWE, they also will be entering the world of Big Data. Cuffel notes that one of the biggest opportunities presented by Big Data is having an increasing amount of clinical evidence on entire populations. We know different types of patients respond differently to treatments. Unfortunately, in clinical trials we often do not have a great amount of patient diversity. RWE allows pharma to better see the effect of medicines on women, African-Americans, Latinos, and other groups that tend to be under-represented in clinical trials.
“RWE will allow us to look at a meaningful level of patient numbers and see exactly what treatments patients are receiving and at what dosage levels,” says Cuffel. “This then allows us to determine which treatments benefit which patient groups the most. We also can look at combinations of treatments, an increasingly important area in oncology that is helping us create the most benefit for patients. That can be done only with large samples of patients.”
“The data we get from study participants in real- world settings allows us to see what they are thinking,” Daley states. “Patients tend to talk about what is important to them. It’s those comments we get from analyzing RWE that help us create better designs for future trials. We also can use that information to better recruit patients and increase the overall level of patient involvement in clinical trials.”
Cuffel adds that RWE can help pharma better understand areas of unmet need, especially for patients receiving treatment with commercial therapies and standard of care. Pharma benefits from learning how to produce better protocols and inclusion/exclusion criteria, which leads to better patient recruitment and, ultimately, patients who are more engaged throughout the study.
Clearly, being able to provide patients with a treatment they are more likely to respond to could be considered the most patient-centric of all approaches. The ability to mine that data and use it in drug development to test therapies on the market against various biomarkers will be an area of great opportunity for both sponsors and patients. According to Cuffel, those abilities also will take the industry to a new level of patient-centricity.
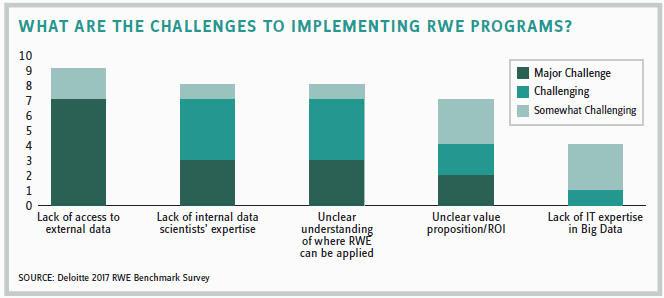
Not Just For Oncology
While RWE seems to be a great fit for oncology studies, its use can span all therapeutic areas. When it comes to the use of RWE, Cuffel believes cardiovascular disease is the area that leads all others. In the cardiovascular field, information for the primary trial endpoints is often available in healthcare claims databases. But in oncology that is not the case. Researchers had to wait for the evolution of more sophisticated clinical data, which now has become a reality.
Daley agrees that RWE is applicable to all therapeutic areas. He notes that in 2012 there were questions surrounding a Sanofi diabetes drug and whether it presented an increased risk of cancer. For that insulin drug, Lantus, Sanofi performed a very large RWE study to understand whether the risk was real. That study involved 12,000 patients and found no association between Lantus and cancer. Three additional studies supported that finding.
The data from any clinical trial can contain collection errors, typos, outliers, and other anomalies that must be cleaned up prior to regulatory submission. The situation can be even more pronounced when dealing with data from the real world. In the real world, you do not have randomization of treatments to patients. A patient will get what their physician prescribes for them, and that treatment is impacted by a wide range of factors. Conversely, the outcome also is going to be affected by a multitude of factors, not just the treatment itself.
“I expect many of the major pharma players to increase their investment in RWE,” says Cuffel. “It will continue to be a growing trend into 2018 and beyond.”
Nevertheless, he’s quick to note that there will be challenges related to how the data is collected and its consistency. “Pharma will need greater expertise in analytics and Big Data, and many companies will need to add qualifi ed analytics groups to correctly review the data. For many companies, RWE is still a new concept, but everyone will see its advantages and get better at implementing it.”
